Selenothrips rubrocinctus (Giard, 1901)
Panchaetothripinae, Thripidae, Terebrantia, Thysanoptera
Figures
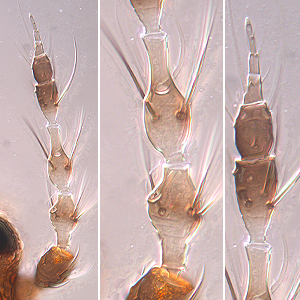
Fig. 1: 8-segmented antenna, segments III and IV with forked sense cone, terminal segments V-VIII
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Head and pronotum
Fig. 4: Meso- and metanotum
Fig. 5: Meso- and metasternum
Fig. 6: Fore- and hind wing, fore wing distal region
Fig. 7: Tergites VI and VII
Fig. 8: Sternites VI and VII
Fig. 9: Tergites VIII-XI
Fig. 10: Adults, male and female
Introduction and recognition
Selenothrips rubrocinctus is a leaf-feeding thrips on cashew, cocoa, mango and many other plants. Both sexes fully winged. Body blackish brown; tarsi and apices of tibiae yellow; antennal segments brown except for III & V yellow in basal half, IV yellow at base and apex, VII & VIII grey; fore wings uniformly dark brown with black setae. Antennae 8-segmented; segments III & IV constricted into neck at base and apex, and with sense cone long and forked, sensorium on VI extending beyond antennal apex, III-V with long dorsal setae (Fig. 1). Head with transverse reticulation, cheeks sharply constricted to basal neck; 3 pairs of ocellar setae, pair III arise on anterior margins of ocellar triangle (Fig. 2). Pronotum with transverse lines of sculpture; no prominent posteroangular setae, but 1 pair of moderately long anteromarginal setae (Fig. 3). Mesonotum transverse reticulate; without median division. Metanotum with median triangle enclosing transverse lines of sculpture; with 1 pair of setae medially and 1 pair of campaniform sensilla (Fig. 4); metathoracic furca elongate and Y-shaped (Fig. 5). Mid and hind tarsi 1-segmented. Fore wing first vein close to or fused to costal vein; fore wing with long costal setae about twice as long as wing width medially; first and second vein with complete row of widely spaced setae; posteromarginal cilia undulated (Fig. 6). Tergites with lateral thirds reticulate; III-VIII with median setae longer than distance between their bases (Fig. 7); VIII with many discal microtrichia and a complete comb of long microtrichia (Fig. 9); tergite X without longitudinal division. Sternites with 3 pairs of long median setae (Fig. 8).
Male similar to female; sternites III-VII with small oval glandular area on antecostal ridge; tergite IX with 3 pairs of prominent stout and thorn-like setae (Fig. 10).
Taxonomic identity
Species
Selenothrips rubrocinctus (Giard, 1901)
Taxonomic history
Selenothrips indicus Bagnall, 1929
Brachyurothrips indicus Bagnall, 1926
Heliothrips (Selenothrips) mendax Schmutz, 1913
Heliothrips (Selenothrips) decolor Karny, 1911
Heliothrips rubrocinctus Franklin, 1908
Physopus rubrocinctus Giard, 1901
Common name
Cacao thrips
Red-banded thrips
Present taxonomic position
Family: Thripidae Stephens, 1829
Subfamily: Panchaetothripinae Bagnall, 1912
Genus: Selenothrips Karny, 1911
Genus description
The genus Selenothrips Karny, 1911
There is only one species recognized in this genus. The area of origin of Selenothrips rubrocinctus is not clear, either Africa or South America, but is now found throughout the tropics. Typical features of this genus are long setae on antennal segments, and segments III and IV constricted apically and basally. Antennal segment III and IV each with a long forked sense cone. 2 rows of long dark stout veinal setae on the uniformly dark brown fore wings. Head constricted to a basal neck, but without a transverse ridge dorsally (Mound & Kibby 1998).
Species description
Typical key character states of Selenothrips rubrocinctus
Coloration and body sculpture
Body color: mainly brown to dark brown
Surface of head, pronotum and fore legs: with heavy, often polygonally reticulate sculpture
Sculptured reticles on head and pronotum: with no internal markings
Antennae
Form of sense cones on antennal segments III and IV: emergent and forked on segments III and IV
Number of antennal segments: 8
Forked sense cone on antennal segment IV: extending to a point at least a third to base of segment V
Terminal antennal segments: VI-VIII forming a single unit
Head
Cheeks shape: constricted to basal neck
Head - occipital ridge dorsally: absent
Head: not prolonged in front of compound eyes (misinterpreted: distinctly prolonged)
Ocelli: present
Prothorax
Pronotal blotch or internal apodeme: absent
Pronotum shape: broadly rectangular
Pronotum surface: with transverse striate sculpture
Mesothorax
Mesonotum: with an incomplete median division
Metathorax
Metanotum with dominant sculptured triangle medially: with weakly defined reticulate triangular area medially
or with dominant sculptured triangle medially
Shape of metathoracic furca: elongate and Y-shaped
Wings
Fore and hind wings: present, more than half as long as abdomen (macropterous)
Fringe cilia arising: from sockets
Fore wing veins: present
Fore- and hind wing surface: covered with microtrichia
Apex of fore wing: with prominent terminal setae
Fore wing anterior margin (costal vein): with setae and cilia but cilia longer than setae
Fore wing costal fringe cilia: arising at anterior margin of wing
Fore wing first vein: close to or fused to costal vein
Fore wing first vein setal row: complete, with setae closely and uniformly spaced
Fore wing second vein setal row: complete, setae uniformly spaced
Fore wing shape: mainly parallel sided or margins run continuously towards each other
Fore wing surface: not reticulate
Fringe cilia on posterior margin near apex: distinctly wavy (undulated)
Length of fore wing costal setae at middle of wing: longer than half or about twice or more as median wing width
Shape of fore wing apex: with mainly posterior margin curved to join anterior margin
Fore wing extreme apex color: dark
Fore wings: uniformly dark brown
Legs
Mid and hind tarsi: with one segment
Color of fore tarsi: pale or yellow, sometimes apical shaded or brown
Abdomen
Tergite II: without specialised cuticle laterally
Tergites IV and V median setal pair: longer than distance between their bases
Tergite VIII to X: without unusually long and stout setae
Tergites: without distinctive tergal sculpture forming a series of arches on the antecostal ridges
Tergite X: not tubular, longitudinally incomplete
Setae on abdominal tergite X: all setae slender

Similar or related species
The heavily dark brown fore wings distinguish this species from most other panchaetothripinae. The genus Selenothrips is similar to the monobasic African genus Xestothrips, with dark fore wings too, but Xestothrips glabratus lacks strong sculpture on the head and pronotum, the occipital ridge on head is conspicuous, the fore wing first vein with the setal row widely interrupted on middle third, and the fore wing costal setae are always at most as long as wing width. Selenothrips rubrocinctus has a strongly sculptured head and pronotum, a indistinct occipital ridge on head, a complete row of evenly placed setae on both veins of the fore wings, and fore wing costal setae at middle of wing are up to twice as long as wing width medially. The shape of the head, legs, and anntennae gives indications of similarity with Caliothrips, but Selenothrips differs in the head constricted posteriorly, the transverse striated pronotum, and the abdominal microtrichia and complete comb on tergite VIII. Just as Selenothrips rubrocinctus, also Panchaetothrips noxius and Monilothrips kempi have fore wing costal setae up to twice as long as wing width, but both species differs by the distinct occipital ridge on head, the pronotum without strong sculpture, light brown fore wings with pale sub-base, the 2-segmented tarsi, and the absence of posteromarginal comb of microtrichia on tergite VIII. Furthermore, Monilothrips kempi has the surface of head not strongly reticulated except for occiptal collar on head, the pronotum with 2 pairs of elongated setae, and the metanotum without dominant or weakly sculptured triangle medially. In Panchaetothrips noxius the fore wing first vein setal row is incomplete and the second vein setal row is absent.
Biology
Life history
Selenothrips rubrocinctus is a slow moving insect requiring prodding to take a few steps across the leaf. The eggs inserted into the leaf tissue; hatching takes about 12-18 days. The larvae have three red-colored bands developed from internal pigmentation. Thus is the reason for the common name ´red-banded cacao thrips´. Larval period lasts 6-10 days and pupal period 3-6 days, so that incubation time from egg to adult can take 3-5 weeks dependent upon seasonal temperatures. Adults, larvae and pupae occur together on leaves (Hill 1983).
Host plants
Polyphagous, mainly on cacao, also on avocado, cashew, guava, mango, orange, pear.
Vector capacity
None identified, but possible mechanical distribution of phytopathogenic fungi and bacteria.
Damage and symptoms
The damage is associated with older leaves, where the lower leaf surfaces are darkly stained, rusty in appearance, and with numerous small, shiny black spots of excreta; leaf edges are curled (Hill 1983).
Detection and control strategies
It is preyed upon by a large assortment of natural predators such as spiders and mites (Wasmannia auropunctata), lacewings (Chrysopidae), predatory thrips (Franklinothrips tenuicornis, Franklinothrips vespiformis), and predatory bugs (Termatophylidea maculata), especially minute pirate bugs (Orius thripoborus) (Chin & Brown 2008; Dennill 1992; McCallan 1943). The wasp parasite Goetheana shakespearei from the Gold Coast and Java attacks many species of thrips but its principle host is Selenothrips rubrocinctus, which it is able to control (Dohanian 1937).
Chemical controls are not always necessary for this thrips, as natural controls are apparently effective most of the time (Denmark & Wolfenbarger 1971).
Additional notes
This species is particularly common on weak or damaged host plants, taking advantage of nutritional abnormalities by feeding on the leaves. Populations increase particularly when plants are water stressed (Fennah 1965).
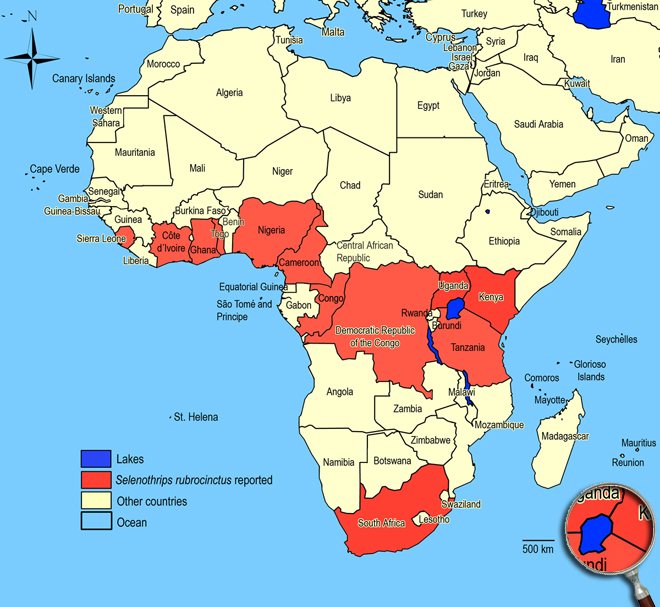
Biogeography
Widespread in tropical and subtropical countries. Cameroon, Congo,
Ghana, Ivory Coast (Le Banco), Kenya (Coastal Province), Nigeria, Sierra Leone, South Africa (KwaZulu-Natal: Port Shepstone, Richards Bay; Mpumalanga: Nelspruit, White River, Brondal), Tanzania,
Togo (Lomé), Uganda (Kampala).
African countries where Selenothrips rubrocinctus has been reported
Occurence of Selenothrips rubrocinctus in East Africa
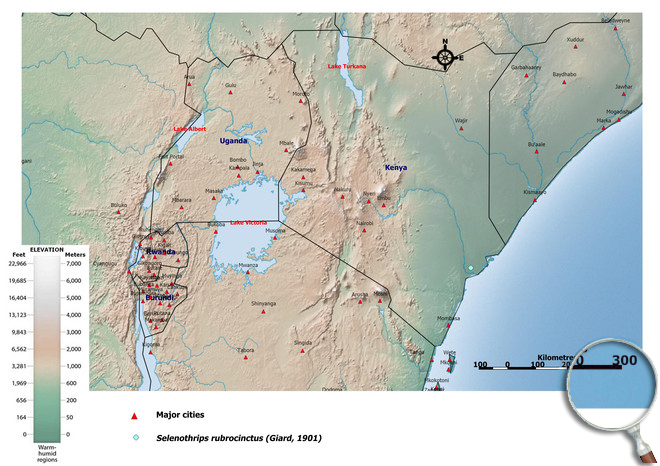
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Click here for locations of Selenothrips rubrocinctus in parts of East Africa.

Bibliography
Ananthakrishnan TN (1971). Thrips (Thysanoptera) in agriculture, horticulture & forestry - diagnosis, bionomics & control. Journal of Scientific and Industrial Research. 30 (3): 113-146
Bagnall RS (1926). Brief descriptions of new Thysanoptera - XV. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 9) 18: 98-114
BoboyeSO (1968). Studies on the biology and chemical control of the red banded cocoa thrips, Selenothrips rubrocinctus Giard. (Thysanoptera: Thripidae), infesting cashew at Okigwi, Eastern Nigeria. Nigerian Entomologist's Magazine. 1: 77-81
Chin D & Brown H (2008). Red-banded thrips on fruit trees. Agnote. 134: 1-3
http://www.nt.gov.au/d/Content/File/p/Plant_Pest/719.pdf
Denmark HA & Wolfenbarger DO (1971). Redbanded thrips, Selenothrips rubrocinctus (Giard) (Insecta: Thysanoptera: Thripidae). Entomology Circular No. 108. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, 4 pp. http://edis.ifas.ufl.edu/in256
Dennill GB (1992). Orius thripoborus (Anthocoridae), a potential biocontrol agent of Heliothrips haemorrhoidalis and Selenothrips rubrocinctus (Thripidae) on avocado fruit in the eastern Transvaal. South African Avocado Growers Association Yearbook 1992. 15: 55-56
http://www.avocadosource.com/Journals/SAAGA/SAAGA_1992/SAAGA_1992_PG_55-56.pdf
Dennill GB & Erasmus MJ (1992). Basis for a practical technique for monitoring thrips in avocado orchards. Crop Protection. 11 (1): 89-91
Dohanian SM (1937). Life history of the thrips parasite Dasyscapus parvipennis Gahan and the technic for breeding it. Journal of Economic Entomology. 30 (1): 78-80
Faure JC (1962). Thysanoptera of Africa - 7. Entomologisk Tidskrift. 83 (1-2): 4-43
Fennah RG (1965). The influence of environmental stress on the cacao tree in predetermining the feeding sites of cacao thrips, Selenothrips rubrocinctus (Giard), on leaves and pods. Bulletin of Entomological Research. 56: 333-349
Franklin HJ (1908). On a collection of thysanopterous insects from Barbados and St. Vincent Islands. Proceedings of the United States National Museum. 33 (1590): 715-730
Giard A (1901). Sur un thrips (Physopus rubrocinctus nov. sp.) nuisible au cacaoyer. Bulletin de la Société Entomologique de France. 15: 263-265
Hill D (1983). Agricultural insect pests of the tropics and their control, (2nd edition). Cambridge University Press, Cambridge, 746 pp
Karny H (1911). Revision der Gattung Heliothrips Haliday. Entomologische Rundschau. 28: 179-182
Karny H (1925). On some tropical Thysanoptera. Bulletin of Entomological Research. 16 (2): 125-142
Kudo I (1992). Panchaetothripinae in Japan (Thysanoptera, Thripidae), 1. Panchaetothripini, the genera other than Helionothrips. Japanese Journal of Entomology. 60 (1): 109-125
Lewis T (1973). Thrips: their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp.
McCallan E (1943). Natural enemies of the cacao thrips. Bulletin of Entomological Research. 34: 313-321
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Mound LA (1968). A review of R. S. Bagnalľs Thysanoptera collections. Bulletin of the British Museum (Natural History), Entomology. Supplement 11: 1-181
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp
Mound LA & Marullo R (1996). The thrips of Central and South America: An introduction (Insecta: Thysanoptera). Memoirs on Entomology, International, Vol. 6. Associated Publishers, Gainsville, 487 pp.
Palmer JM (1990). Identification of the common thrips of Tropical Africa (Thysanoptera, Insecta). Tropical Pest Management. 36 (1): 27-49
Palmer JM, Mound LA & du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Pitkin BR & Mound LA (1973). A catalogue of West African Thysanoptera. Bulletin de ľInstitut Fondamental ďAfrique Noire, Série A. 35 (2) 407-449
Priesner H (1952). On some Central African Thysanoptera. Bulletin de ľInstitut Fondamental de ľAfrique Noire. 14 (3): 842-880
Russell HM (1912). The red-banded thrips (Heliothrips rubrocinctus Giard). Bulletin, USDA, Bureau of Entomology. 99 (2): 17-29
Wilson TH (1975). A monograph of the subfamily Panchaetothripinae (Thysanoptera: Thripidae). Memoirs of the American Entomological Institute. 23: 1-354
zur Strassen R (1980). Thysanopterologische Notizen (5) (Insecta: Thysanoptera). Senckenbergiana Biologica. 60 (3-4): 191-202
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California